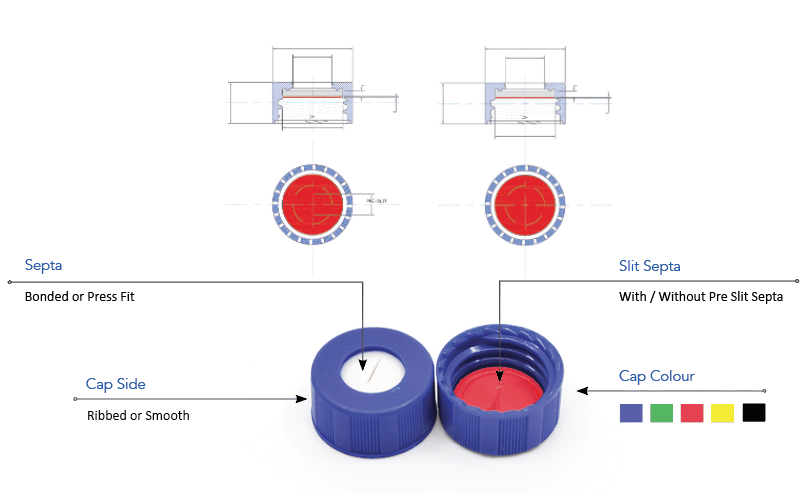
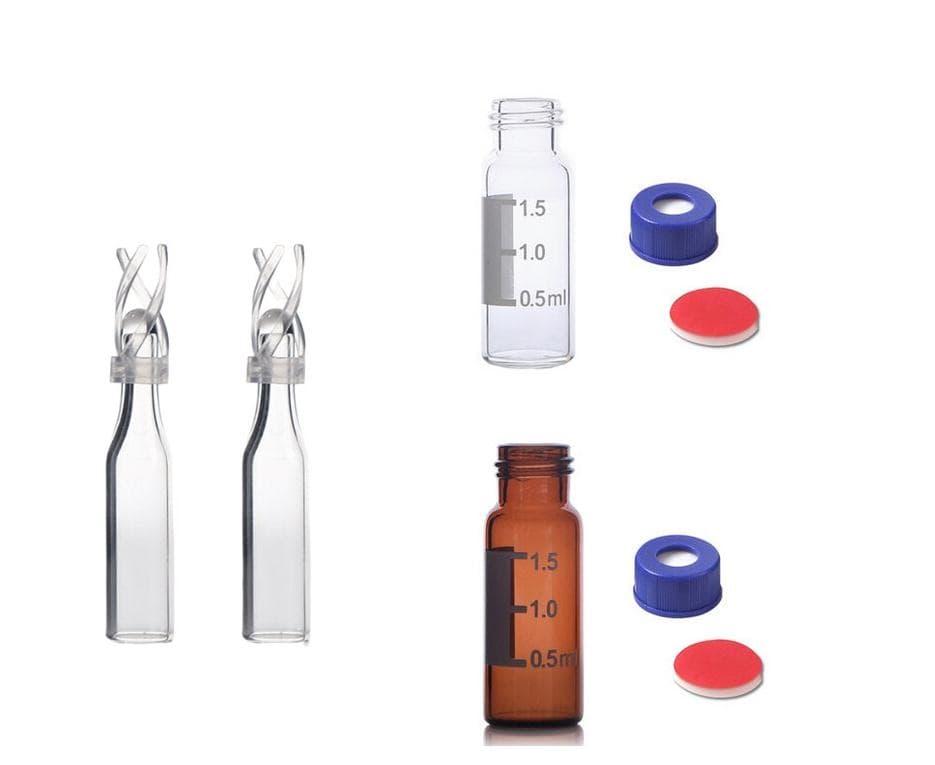
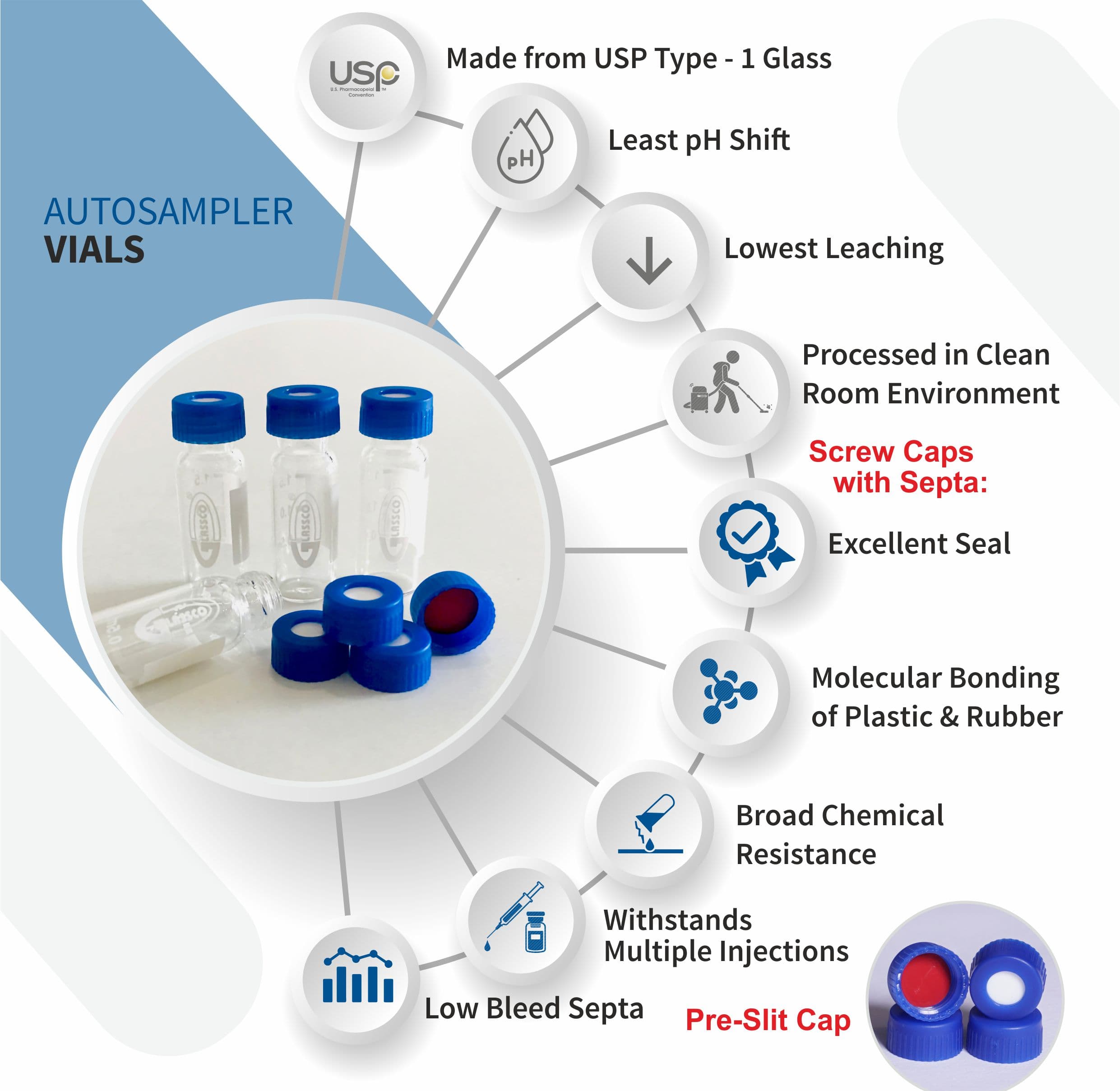
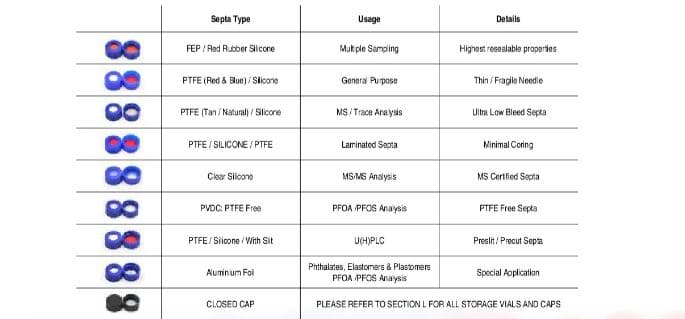
-
Data quality is a broader category of criteria that organizations use to evaluate their data for accuracy, completeness, validity, consistency, uniqueness, timeliness, and fitness for purpose. Data integrity focuses on only a subset of these attributes, specifically accuracy, consistency, and completeness. It also focuses on this more from the
-
Each quality measure focuses on a diferent aspect of healthcare, such as processes, patient health outcomes, patient perceptions, and organizaional structure and/or systems. Quality measures help by measuring these key aspects of healthcare, which are chosen because they: Are associated with the ability to provide high-quality healthcare, and/or
-
Mar 1, 2021 · Quality assurance measures ensure competence and reliability in the laboratories procedures from patient specimen receipt (pre-analytical phase), specimen handling and testing (analytical phase), to the analysis and reporting of patient results (post-analytical phase).
-
with objective measures and quantitative research designs. Quantitative researchers, in contrast to qualitative researchers, are comfortable with an orientation toward understanding the objective world via experimental designs that test hypotheses born from theories and result in statistical gener-alizations that apply to a population at large.
-
Sep 4, 2020 · 8.1 Introduction. Quality control is an essential component of international large-scale assessment. Rigorous quality control processes help ensure high quality data and facilitate cross-country comparisons of study results. Although quality control can and does exist at all stages, including before, during, and after data collection, the term
-
Aug 27, 2023 · Add your perspective. The fourth step to ensuring data quality and accuracy is to review and communicate your data results in a clear and concise manner. You should check your data results for any
-
The laboratory shall have quality control procedures for monitoring the validity of tests and calibrations undertaken. The resulting data shall be recorded in such a way that trends are detectable and, where practicable, statistical techniques shall be applied to the reviewing of results.
-
Sep 13, 2023 · Data quality management is a set of practices that aim at maintaining a high quality of information. It goes all the way from the acquisition of data and the implementation of advanced data processes to an effective distribution of data. It also requires a managerial oversight of the information you have.
-
Dec 13, 2018 · 4. Monitor and control data. Data quality management is not a one-time effort, rather a non-stop process. You need to regularly review data quality policies and rules with the intent to continuously improve them. This is a must, as the business environment is constantly changing.
-
Figure 4.2 shows the correlation between two sets of scores of several university students on the Rosenberg Self-Esteem Scale, administered two times, a week apart. The correlation coefficient for these data is +.95. In general, a test-retest correlation of +.80 or greater is considered to indicate good reliability.
-
Data Quality Management (DQM): A formal quality improvement process that ensures the validity and integrity of data during collection, aggregation, storage and analysis. Quality Assurance (QA): For the purposes of this course, QA a is planned and systematic activity
-
Aug 27, 2023 · Define. Start by defining the quality standards that you need to control and improve. In some industries, many quality standards are determined by a regulatory agency. For example, quality control begins with food suppliers in the food industry. However, restaurant owners also have quality control standards to maintain, including
-
Laboratory Quality Standards and their Implementation ix Scope and objectives The objective of this document on Laboratory Quality Standards is for it to be used as a guideline for evaluation, development and implementation of quality systems and standards for health laboratory services. The internationally
-
Jul 28, 2019 · However, if a company can manage the data quality of each dataset at the time when it is received or created, the data quality is naturally guaranteed. There are 7 essential steps to making that happen: 1. Rigorous data profiling and control of incoming data. In most cases, bad data comes from data receiving.
-
internal quality control and external quality control, and participation in external proficiency testing (PT) programmes. All the laboratory’s training, calibration and diagnostic processes must be documented and retained for audit purposes. Even for smaller laboratories that may not have the resources necessary to implement accredited