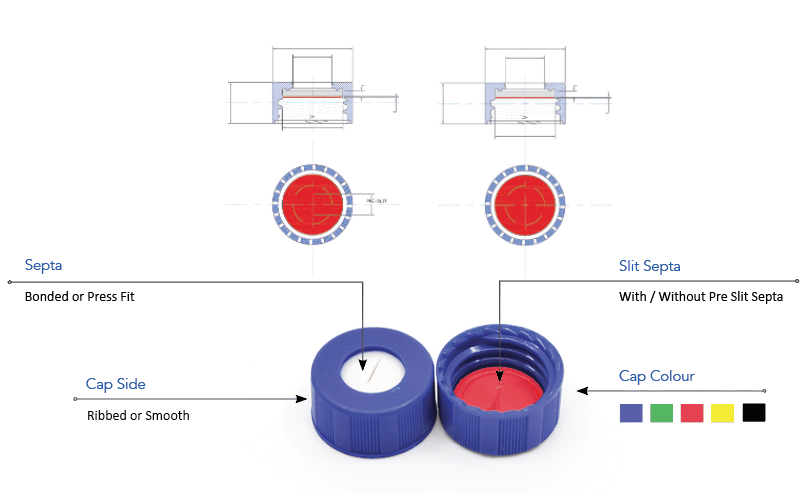
-
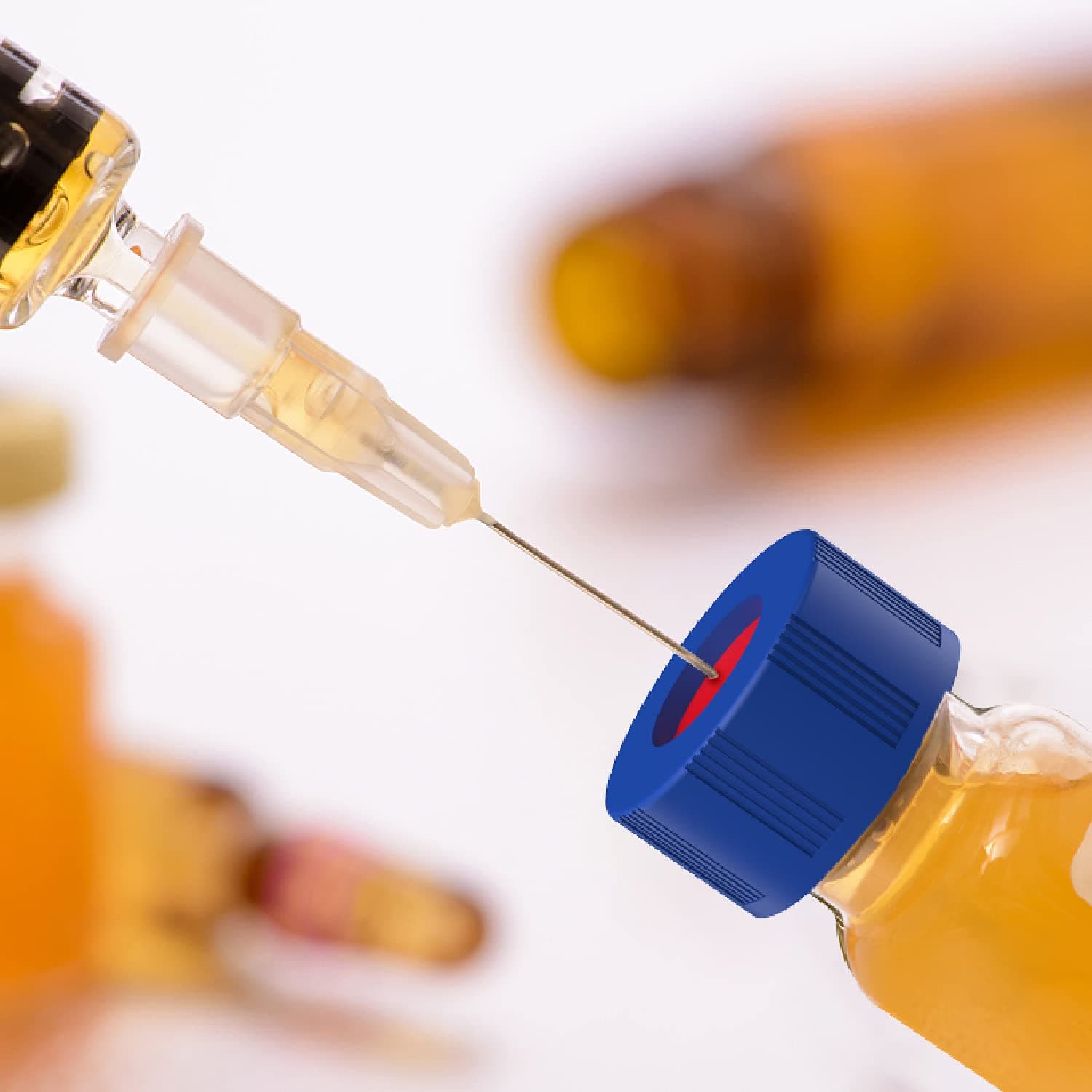
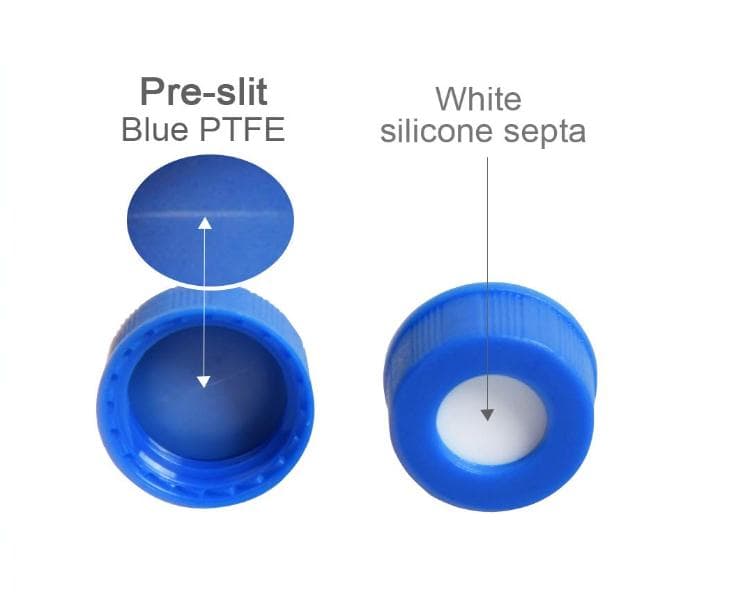
When robustness and reproducibility are key SureSTART Level 2 vials have very low metal contents and leachables to ensure that they conform to the high standards you need to meet your regulatory requirements. The septa are conditioned to provide a very low level of chromatographic extractables.
-
Technical Support Customer Service. Use Aijiren Tech™ SureSTART™ Level 1 Polyspring Plastic Inserts with 2 mL screw, crimp, or snap vials to maximize injection volume when analyzing <2 mL samples. The integral polyspring at the bottom of the insert centers the insert in the vial, ensures a good seal with the vials’ cap, and provides
-
Use Aijiren Tech™ SureSTART™ Performance Level 3 Inserts with 2 mL vials when analyzing <2 mL samples to maximize the injection volume. These glass inserts have a small total volume capacity of <400 μL, with a minimum residual volume of 2 µL. When placed and sealed inside a 2 mL vial, microsamples can be used in conventional
-
Jun 18, 2021 · This method, which is available for assay and dissolution studies, was fast, environmentally friendly, and more cost-effective than the earlier published methods. 24,25,26 In this study, DOE was adapted to the robustness parameter of the analytical method for determining amlodipine and enalapril together. DOE principles were used in the method
-
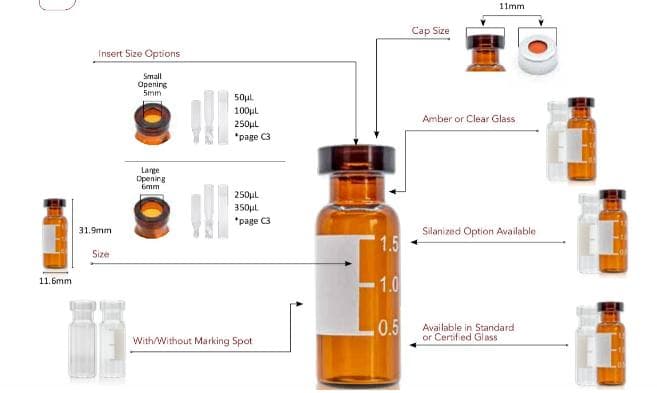
Technical Support Customer Service. Use Performance Level 1 Aijiren Tech™ SureSTART™ 2 mL Glass Screw Top Vials for everyday chromatography analysis. They provide an affordable choice and can be used with all GC and HPLC/UHPLC instrument types. These 2 mL glass screw top vials are Specification Certified to ensure autosampler
-
Nov 8, 2021 · Recommended Best Practices for Lyophilization Validation 2021 Part II is a comprehensive guide for the pharmaceutical industry on how to perform and document the validation of lyophilization processes. It covers topics such as protein concentration measurement, stability testing, and regulatory requirements. This article is the second part of a two-part series published in the Journal of
-
Jul 11, 2014 · A robustness test is designed to show the reliability of a method response as different parameters are varied. It is the first stage of a robustness test to decide on which parameters should be tested and by how much to vary them. The factors fall broadly in one of two areas: Operational factors (analytical procedure/operating procedure)
-
Mar 1, 2023 · Enhance method understanding and identify acceptable operating conditions through method robustness testing using a multivariable DoE with Empower Method Validation Manager (MVM) Software Automate creation of chromatographic methods for robustness testing with Empower Sample Set Generator (SSG) tool
-
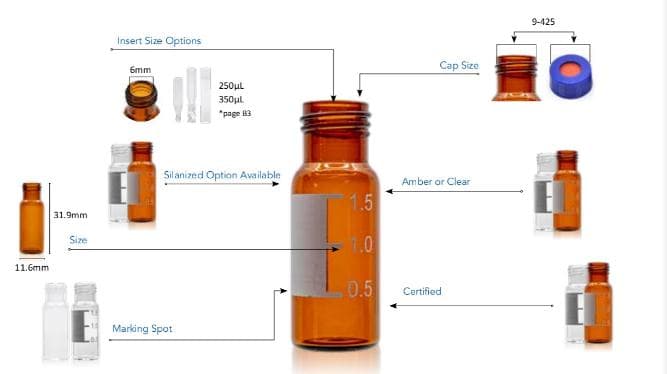
Select Level 2 products for high throughput applications, when robustness and reproducibility are key. Our kits for scintillation include 20 mL glass screw vials and closed top polypropylene caps lined with polypropylene foil septa.
-
6912 Well Plates, × 0.5 mL or 0.7 mL Vials, 972 × 2 mL Vials, 360 × 10 mL or 20 mL Vials: Injection Mode: 8 Fully Customizable Method-Specific Injection Mode Presets: Injection Speed: Selectable from 0.1 μL/s to 2000 μL/s: Injection Volume: Description: TriPlus RSH SMART configured for liquid injection on extended X-axis length.
-
The European Pharmacopoeia (Ph. Eur.) hydrolytic resistance method for glass vials is routinely used to screen pharmaceutical glass vial supply. In an effort to better understand and control the factors affecting method precision and robustness, several potential sources of variability in the Ph. Eur. alkalinity method have been studied for 3
-
Accuracy is the closeness of the test results obtained by the method to the true value. Accuracy has been performed by addition of standard in the test sample at 50%, 100% and 150 % of specification level and then determining the content. Robustness The robustness of the analytical procedure is the measure of method capacity to remain
-
Jan 27, 2021 · Materials. All vials were obtained from SGD S.A. (Puteaux, France). Three different types of molded vials with a nominal fill volume of 20 mL were used in this study: 20-mL vials manufactured by a BB process (“20-mL BB”), by a PB process with the same manufacturing mold as the BB vials (“20-mL PB1”), and by a PB process with from a freeze-drying perspective optimized geometrical
-
Report Method ID: 55375537 10/3/2018 1:14:06 PM US/Pacific CPR 20994 ADR_____ Result Id 3864 S A M P L E I N F O R M A T I O N Sample Name: Ref Std, Prep 1 Acquired By: pnguyen Sample Type: Unknown Sample Set Name: CPR20518_050718_01_GMP37 Vial: 5 Acq. Method Set: APL_GTM11_LC44 Injection #: 1 Processing Method: APL_PM_PDN
-
When robustness and reproducibility are key. SureSTART Level 2 vials have very low metal contents and leachables to ensure that they conform to the high standards you need to meet your regulatory requirements. The septa are conditioned to provide a very low level of chromatographic extractables.