-
Mar 3, 2021 · qPCR and qRT-PCR. qPCR and qRT-PCR have become mainstream life science technology for the quantitation of nucleic acids. 22 The focus of this paper is to mainly describe qPCR analysis of DNA vectors as an example to discuss the approach of method development, validation, and sample analysis. qRT-PCR analysis of the RNA viral genome or vector-derived gene expression are discussed briefly later
-
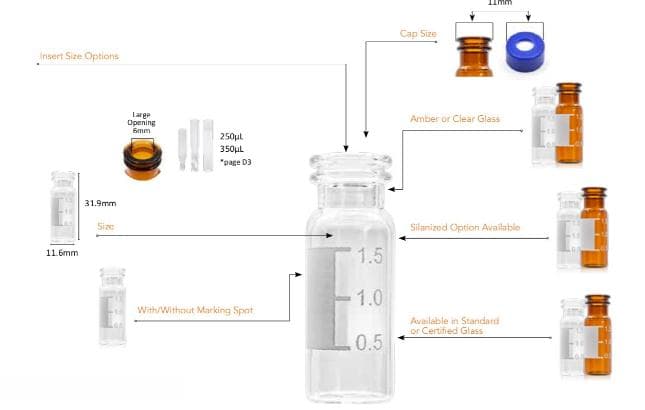
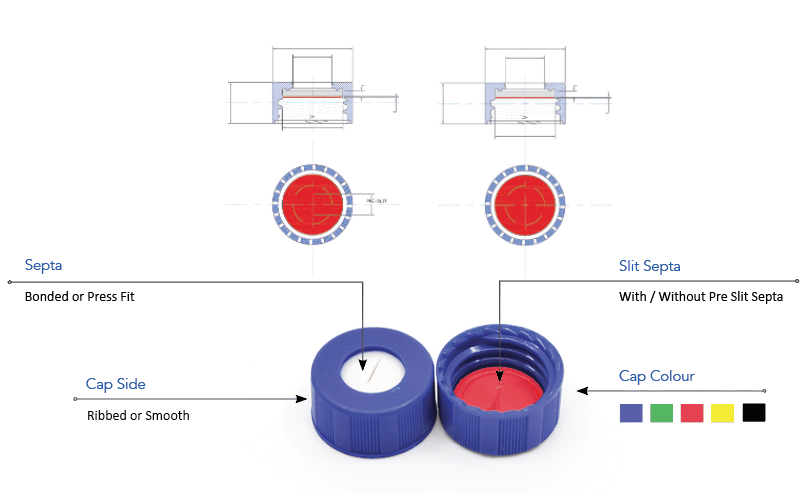
Analytical Vials. Vials play a significant role in analytical analysis and result reproducibility. Vials must be inert and free of extractables or leachables to prevent affecting results. Using certified, application-specific, contaminant-free vials can significantly reduce risk. We offer a broad spectrum of Supelco ® products including
-
A vial-in-vial method is used, in which the volatile agent is placed in a small inner vial, and the inner vial is capped with a layer of fabric or film to be tested. The agent permeates from the inner vial into an outer headspace vial. The instrument samples the vapor in the outer vial by sampling it and injecting it into the GC for analysis.
-
Headspace vials often come in larger volumes than the 2-mL vials used in many liquid sampling systems. Common offerings are 10-mL, 20-mL and 22-mL capacities. Larger vials accommodate larger sample volume and/or a larger headspace above that sample.
-
Explore all our special offers, all in one place. Temporary Price Reductions. Save with limited-time price reductions across select products and categories. View All Special Offers and Programs. Chemical Structure Search Help & Support. Aijiren Tech Scientific. Products. Test Tubes, Vials, Caps and Closures. Tubes.
-
of water, cap the vial, and mix. Prepare the test stock solution and the test solution as follows: • Test stock solution: Transfer 250 mg of the article under test to a 25mL flask, dissolve, bring to volume with water, and mix. • Test solution: Transfer 5 mL of Test Stock Solution to a 20 mL HS vial; add 1 mL of water, cap the vial, and mix.
-
the process used in day-to-day operation of the method, thus making comparison of the two processes difficult. This study presents a fully automated gravimetric solution to prepare all solutions for a calibration or linearity test independently and without error-prone manual interference. All solutions prepared are immediately
-
8 X3.2 mL vials 2 X 10 ng control standard endotoxin 3 X 30 mL LAL reagent water: R1708K: Endochrome-K™ (sensitivity= 50-0.005 EU/ml) 3.2 mL vials: R17100: Endpoint chromogenic kit (140 tests) 5 X 1.4 mL vials of chromogenic LAL 1 X 10 mg vial of chromogenic substrate S-2423 2 X 2 ng vials of endotoxin 2 X 30 mL vials of LAL reagent water
-
There are three common methods, below is a general selection guide of choosing which method to use: Methods Gel-clot Chromogenic Turbidimetric Maximum Sensitivity 0.03 EU/ml 0.01 EU/ml 0.001 EU/ml Additional Material Required Non-circulating water bath or dry bath incubator A microplate reader (an incubating reader is required for the kinetic
-
Consistent vial-to-vial and lot-to-lot performance. High recovery vials for limited sample volumes. Compatible with all autosamplers and designed to fit a range of caps. Complete selection of vial sizes and materials with combination packs for easy ordering.
-
Linearity This method demonstrates excellent linearity of analyzing AAV8 samples from 1X1010GC/mL to 1.6X 1014GC/mL by plotting peak area of VP3 to sample titers (Figure 12). The R2 is 0.9989. Conclusions Presented in this technical note is a CE-SDS-LIF method for assessing the purity of Adeno Associated Virus (AAV) viral
-
Sep 3, 2020 · Set up the instrument as per the testing conditions. Ensure that, the column is conditioned before injecting the sample. Inject the all five linearity standard solution in duplicate and record the area of principal peak in observation table. Plot a linearity curve of concentrations Vs corresponding mean area, using least square method.
-
Guidance 006 Analytical Test Method Validation - Linearity, Range and Specificity. for evaluating linearity. The determination of linearity should be done using the method of quantification specified in the procedure. Evaluation of linearity for TLC may be performed by visual examination of the plate, if this is consistent with the method of
-
Linearity Linearity is the ability of the method to obtain the test results which are directly proportional to the concentration (amount) of analyte in the sample. The linearity study was carried for solvents (Methanol, Ethanol, Acetone, Methylene dichloride, Hexane, Chloroform, Ethyl acetate) from LOQ concentration to 150% of specification level.
-
LAL Techniques and Methods Offered: Testing techniques: • Gel-clot technique: based on gel formation • Turbidimetric technique: based on the development of turbidity • Chromogenic technique: based on the development of color after cleavage of a synthetic peptide-chromogen complex. Testing methods: • Method A: Gel-clot method (limit test)