-
LAL Techniques and Methods Offered: Testing techniques: • Gel-clot technique: based on gel formation • Turbidimetric technique: based on the development of turbidity • Chromogenic technique: based on the development of color after cleavage of a synthetic peptide-chromogen complex. Testing methods: • Method A: Gel-clot method (limit test)
-
The selection of non-clinical and clinical studies is product-driven, i.e. a strategy for comparability testing should be chosen that best predicts or detects clinically relevant differences with sufficient accuracy. This guideline replaces guideline the guideline on comparability of medicinal products containing
-
Jun 7, 2021 · Biologics companies spend a lot of time and money improving their cell lines’ genetic characteristics and expression mechanisms. But cell-bank testing incurs large expenses. Complete safety and characterization testing costs can approach US$200,000. Biologics companies respond differently to those cost concerns.
-
Centrifugation: When plasma is required, or when not using a serum gel separator tube, follow these instructions: Draw 12 mL of whole blood for each 5 mL of serum or plasma needed. Collect in an appropriate collection tube. Centrifuge for at least 15 minutes at 2200-2500 RPM. Pipette the serum or plasma into a clean plastic screw-cap vial and
-
8 X3.2 mL vials 2 X 10 ng control standard endotoxin 3 X 30 mL LAL reagent water: R1708K: Endochrome-K™ (sensitivity= 50-0.005 EU/ml) 3.2 mL vials: R17100: Endpoint chromogenic kit (140 tests) 5 X 1.4 mL vials of chromogenic LAL 1 X 10 mg vial of chromogenic substrate S-2423 2 X 2 ng vials of endotoxin 2 X 30 mL vials of LAL reagent water
-
mM) filled into 6 mL SCHOTT vials using a fill volume of 2 mL. The formulation was freeze dried and the vials were stoppered using either WEST 4416/50 (high moisture) or DAIKYO D777 (low moisture) stoppers. The lyophilized solids were placed in desiccators that were controlled at three relative humidity (RH) levels at ambient temperature. The RH
-
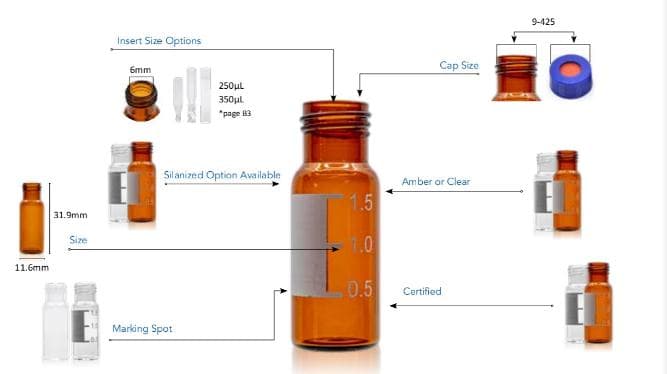
What are the HPLC Vial Micro-inserts? 1-4mL Autosampler Vials for HPLC, UPLC, GC 1.5mL 8-425 Screw Neck Vial ND8 1.5mL 9mm Short Thread Vial ND9 1.5mL 10-425 Screw Neck Vial ND10 1.5mL 11mm Snap Ring Vial ND11 1.5mL 11mm Crimp Ring Vial ND11 4mL 13-425 Screw Neck Vial ND13 1mL Shell Vial Micro-Inserts Vial Racks Hand Crimper, Decrimper
-
Identify methods where a combined reference standard wouldn't be needed. Two different curves. Depends on the method. How are reference standards are aliquotted to determine heterogeneity. Done by hand Will take vials from beginning, middle, and end to perform in the testing. Early phase - 3000 - 4000 Later Phase - 10,000
-
Oct 2, 2014 · A risk-based approach is recommended for analytical method comparability for HPLC assay and impurities methods. To ensure the quality of drug products and patient safety, pharmaceutical companies need to manage and justify changes in chemistry, manufacturing, and controls (CMC). Analytical methods are integral parts of CMC, and some common
-
Nov 19, 2009 · The challenging task of setting appropriate acceptance criteria for assessment of method equivalence is a critical component of satisfying these requirements. The use of these criteria will support changes made to methods across the product lifecycle.
-
Dec 23, 2020 · With the growth of gene and cell therapies, there is an increased need to characterize container closure systems (CCS) at the low temperatures experienced during their lifecycles: ultra-low (-80°C) for genes and cryogenic (-180°C) for cells. West’s Integrated Solutions platform includes the state-of-the-art container closure integrity (CCI
-
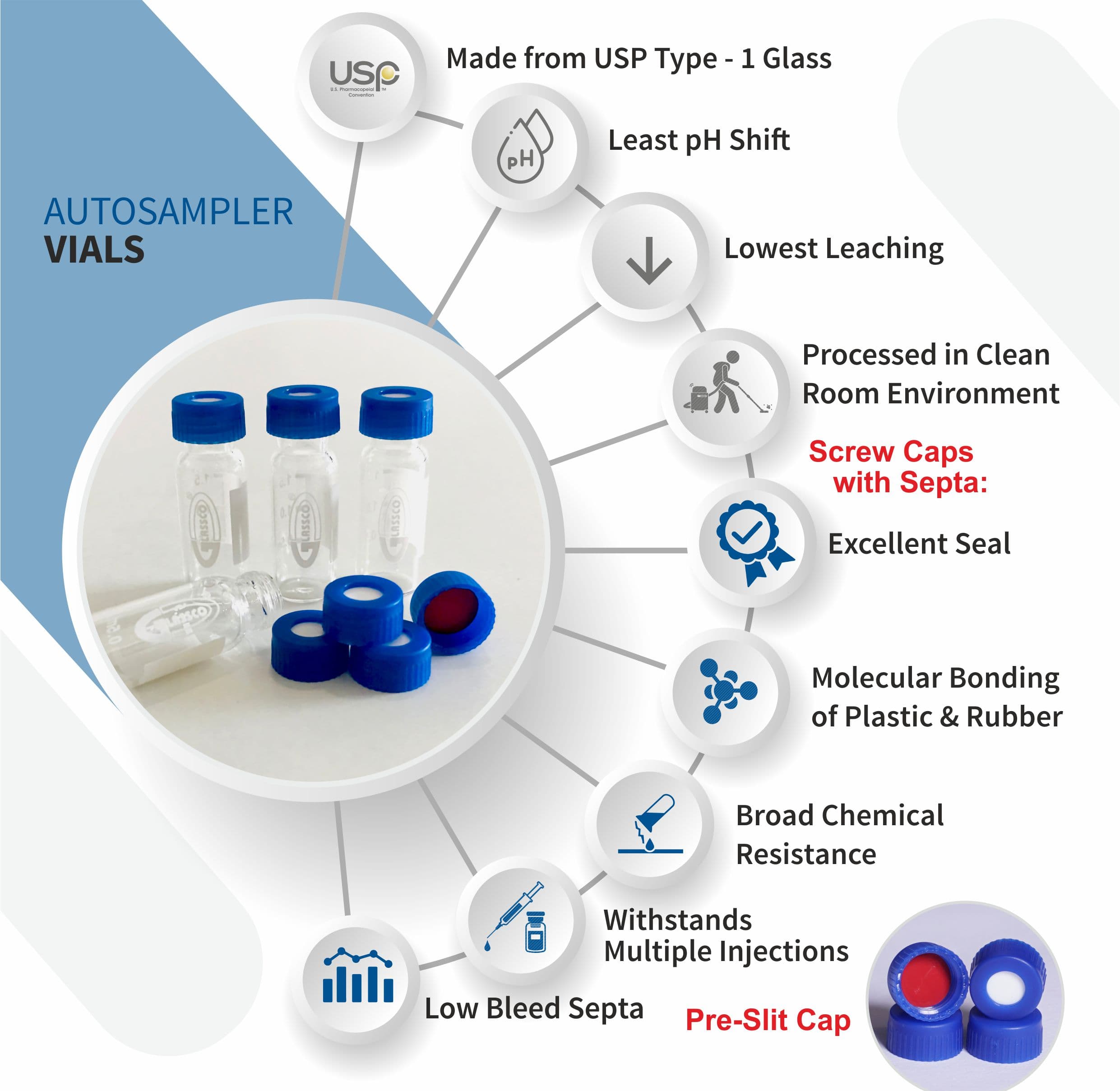
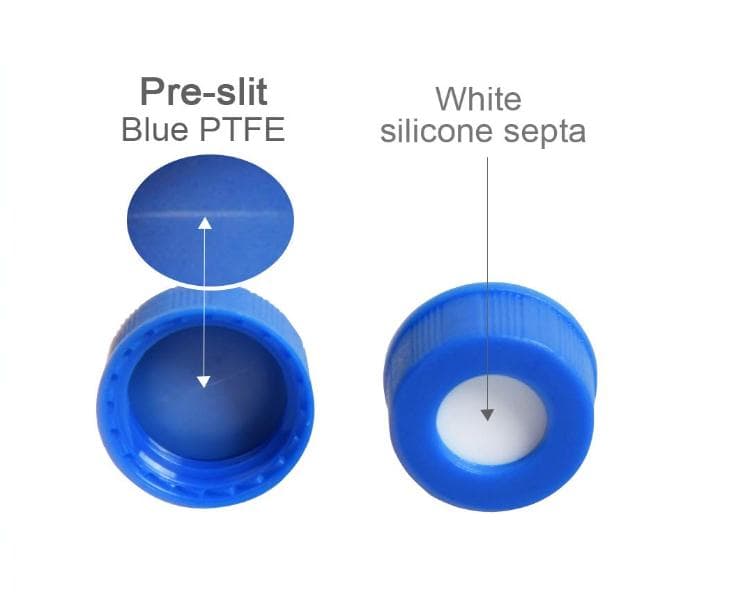
Analytical Vials. Vials play a significant role in analytical analysis and result reproducibility. Vials must be inert and free of extractables or leachables to prevent affecting results. Using certified, application-specific, contaminant-free vials can significantly reduce risk. We offer a broad spectrum of Supelco ® products including
-
Product Packaging. Pyrotell lysate is available in single test vial (STV), 2 mL or 5 mL fill sizes. The 2 mL and 5 mL vials are sold individually. STV’s are sold in packs of ten vials. Control Standard Endotoxin (CSE) is provided separately with a Certificate of Analysis, specific to the Pyrotell lysate lot with which it will be used. Multi-Test.
-
Mar 9, 2021 · Stability assays are used throughout each product’s life cycle, beginning with development and performance of comprehensive and specific stability protocols during preclinical development and early clinical phases. Under the quality by design (QbD) paradigm, stability is part of a biotherapeutic’s quality target product profile.
-
Feb 1, 2008 · In assessments of comparability for postapproval manufacturing changes, the testing requirements often can be limited to physical, chemical, and biological testing. Figure 1: () Risk-Based Approach: Any modification to the method of manufacture for a drug substance carries some risk of causing adverse effects either to the physical properties