-
A Validated HPLC Method for Determining Residues of a Dual Active Ingredient Anti-Malarial Drug on Manufacturing Equipment Surfaces. J. Pharm. Biomed. Anal. 37 (3), 461–468 (2005). M.J. Nozal, et al. Development and Validation of an LC Assay for Sumatriptan Succinate Residues on Surfaces in the Manufacture of Pharmaceuticals. J. Pharm. Biomed.
-
in chromatographic and obviously the spectroscopic analyses of many sample types, was omitted. Many of the techniques covered, however, such as ion exchange SPE, liquid-liquid extraction and microwave-assisted extraction are equally applicable to inorganic samples for further analysis by ion chromatography or for spectroscopic measurements. Preface
-
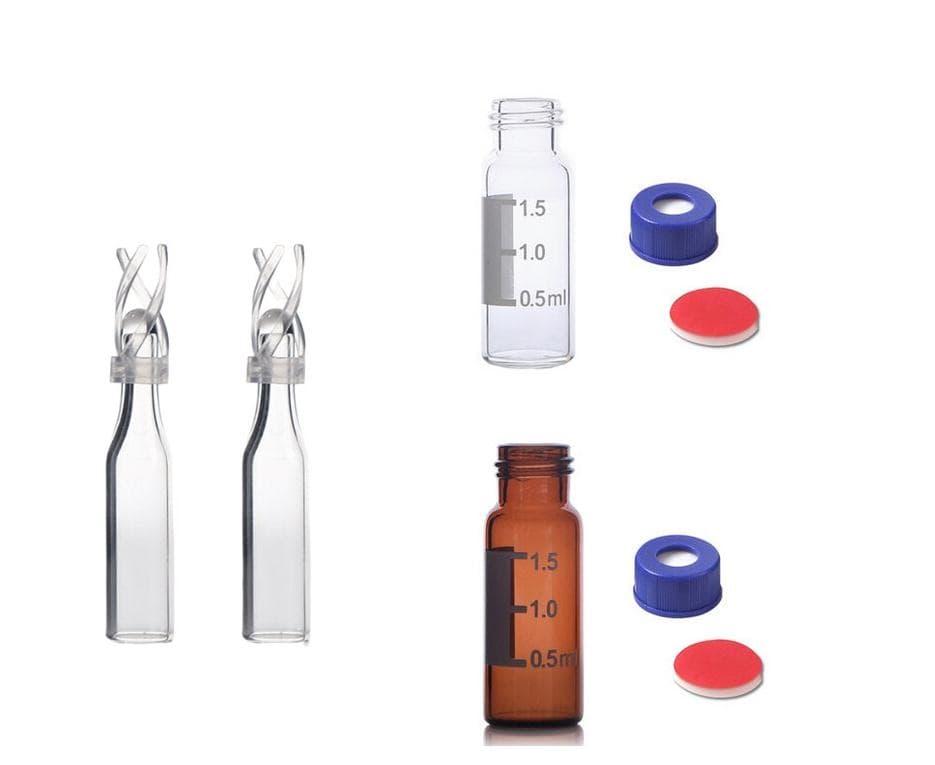
Aijiren's 2 mL screw top vials are constructed from Type 1 borosilicate glass, with low metal content, to protect your sample from destabilizing or leaching. Our 2 mL glass vials are available in both amber and clear versions. The associated 9 mm vial screw cap comes with your choice of septa constructed from either PTFE/silicone, PTFE/silicone
-
Jun 22, 2019 · 1. Pour and dry the liquid in the chromatographic sample bottle. 2. 2. Pour dry after this as the alcohol enters the 1.5mL vial easily and is intersoluble with most organic solvents. 3. Pour in water and wash with ultrasonic twice. 4. Pour the liquid in the bottle and bake at 110 ° c for 1~2 hours. Do not bake at high temperature.
-
Analytical Vials. Vials play a significant role in analytical analysis and result reproducibility. Vials must be inert and free of extractables or leachables to prevent affecting results. Using certified, application-specific, contaminant-free vials can significantly reduce risk. We offer a broad spectrum of Supelco ® products including
-
Dec 26, 2019 · High-performance liquid chromatography (HPLC) has been widely used for years as an analytical method and is a key tool for the separation and analysis of pharmaceutical drugs, for drug monitoring
-
Aijiren's autosampler vials are available as either snap, screw or crimp in style. Most users will be familiar with the main 2 mL vial being the HPLC vial or LC/MS vial; sometimes being referred to as a 1.5 mL vial. We have also designed a range of 2 mL GC vials and GC/MS vials for your gas chromatography applications.
-
Find 2 ml hplc vial and related products for scientific research at MilliporeSigma
-
Jan 5, 2022 · The developed GC method fulfills validation requirements and therefore could be applied generally for determination of residual solvents in 18 F- and 11 C-radiopharmaceuticals. Since the synthesis routes of these radiotracers are universally used, the impurity profile will be similar, and the proposed analytical procedure could be successfully
-
Blanks in Method Validation Eurachem Supplement MV Blanks 2019 Page 4 2 Types and uses of blanks in method validation 2.1 Calibration blank Section 6.3 of the Guide addresses the performance characteristic working range. When determining the working range of the instrument (that is the range of
-
Validation of the method was performed by means of Bias, Linearity, LOD, Selectivity, Specificity, Precision, Robustness and Intermediate precision. The method showed an excellent linearity with correlation coefficient (r 2 = 0.993) was observed in the range from 0.1 to 3.5 mg/mL of ethanol. The percent recovery value were between 91.0 and 109.
-
When your instrument, sample handling, and methodology is pushing the limits, your chromatography vials and seals can’t be the broken link in the chain. Ensuring quality in every part of your workflow, including vials and closures, is critical to success. See the difference in Aijiren Tech Certified Vials and Closures.
-
Jul 21, 2022 · For the quantification of both acidic and neutral cannabinoids, methods based on high performance liquid chromatography (HPLC) 16, thin layer chromatography (TLC) 17, HPLC coupled with mass
-
This step is the most time-consuming part of method development and often requires expert knowledge to perfect. Robustness testing. Done to determine the impact of changing parameters of the separation method. Optimizing robustness is important for many method development and validation processes. Method validation. The industry-specific
-
Aijiren 2ml 10mm chromatography amber vials are constructed from Type 1 borosilicate glass, with low metal content, to protect your sample from destabilizing or leaching. The associated 2ml 10mm chromatography amber vials and closures come with your choice of septa constructed from either PTFE silicone or natural red rubber. Vial Dimension: 11.